Injectables
Botox® & Fillers
At Women4Women, we offer non-surgical cosmetic procedures for patients who wish to maintain their looks, or turn back the clock, without the risks of traditional plastic surgery. Options for treatment include FDA-approved fillers (all Juvéderm® products including Voluma™ and Volbella™) and neuromodulators including BOTOX®. If you're ready for a new look, schedule a consultation for facial contouring with Dr. Reidy.
BOTOX® Cosmetic
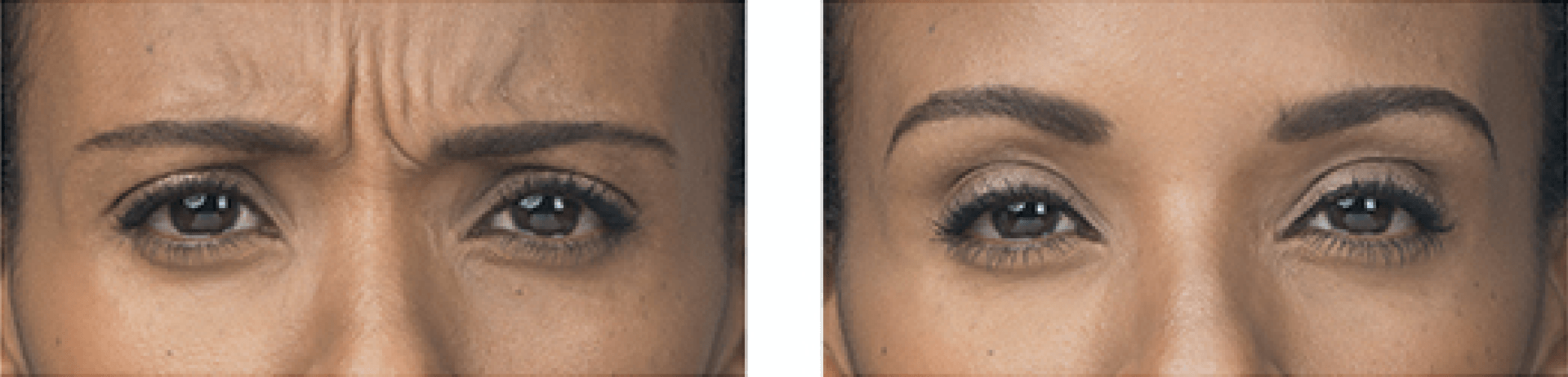
BOTOX® Cosmetic is a prescription medicine that is injected into muscles and used to temporarily improve the look of moderate to severe forehead lines, crow's feet, and frown lines between the eyebrows in adults. Contact our office today to schedule a consultation, and find out if BOTOX® Cosmetic is right for you.
JUVÉDERM® Fillers
The gang’s all here! Smooth lines with JUVÉDERM® VOLLURE™ and JUVÉDERM® XC. Volumize for lift with JUVÉDERM® VOLUMA™ XC. Plump lips with JUVÉDERM® Ultra XC and JUVÉDERM® VOLBELLA™ XC. Contact our office to book a consultation with Dr. Reidy, our JUVÉDERM® specialist, today!
BOTOX® FAQs & Safety Information
- What is BOTOX® Cosmetic?
BOTOX® Cosmetic is the first and only FDA-approved product to temporarily improve both moderate to severe frown lines between the brows and crow’s feet lines around the sides of the eyes in adults.
- How does BOTOX® Cosmetic work?
BOTOX® Cosmetic is the first and only FDA-approved product to temporarily improve both moderate to severe frown lines between the brows and crow’s feet lines around the sides of the eyes in adults.
- How is BOTOX® Cosmetic (onabotulinumtoxinA) administered?
BOTOX® Cosmetic targets one of the underlying causes of frown lines and crow’s feet—the repeated muscle contractions from frowning and squinting over the years. Your doctor will inject these muscles with BOTOX® Cosmetic to temporarily reduce muscle activity. You’ll begin to notice a visible smoothing of these lines.
- Will BOTOX® Cosmetic make me look like I've had work done?
To temporarily improve moderate to severe crow’s feet, your doctor will inject BOTOX® Cosmetic into the muscle surrounding the sides of your eyes—called the orbicularis oculi.
For temporary improvement of moderate to severe frown lines, BOTOX® Cosmetic is injected into 2 muscles—the corrugator and procerus muscles.
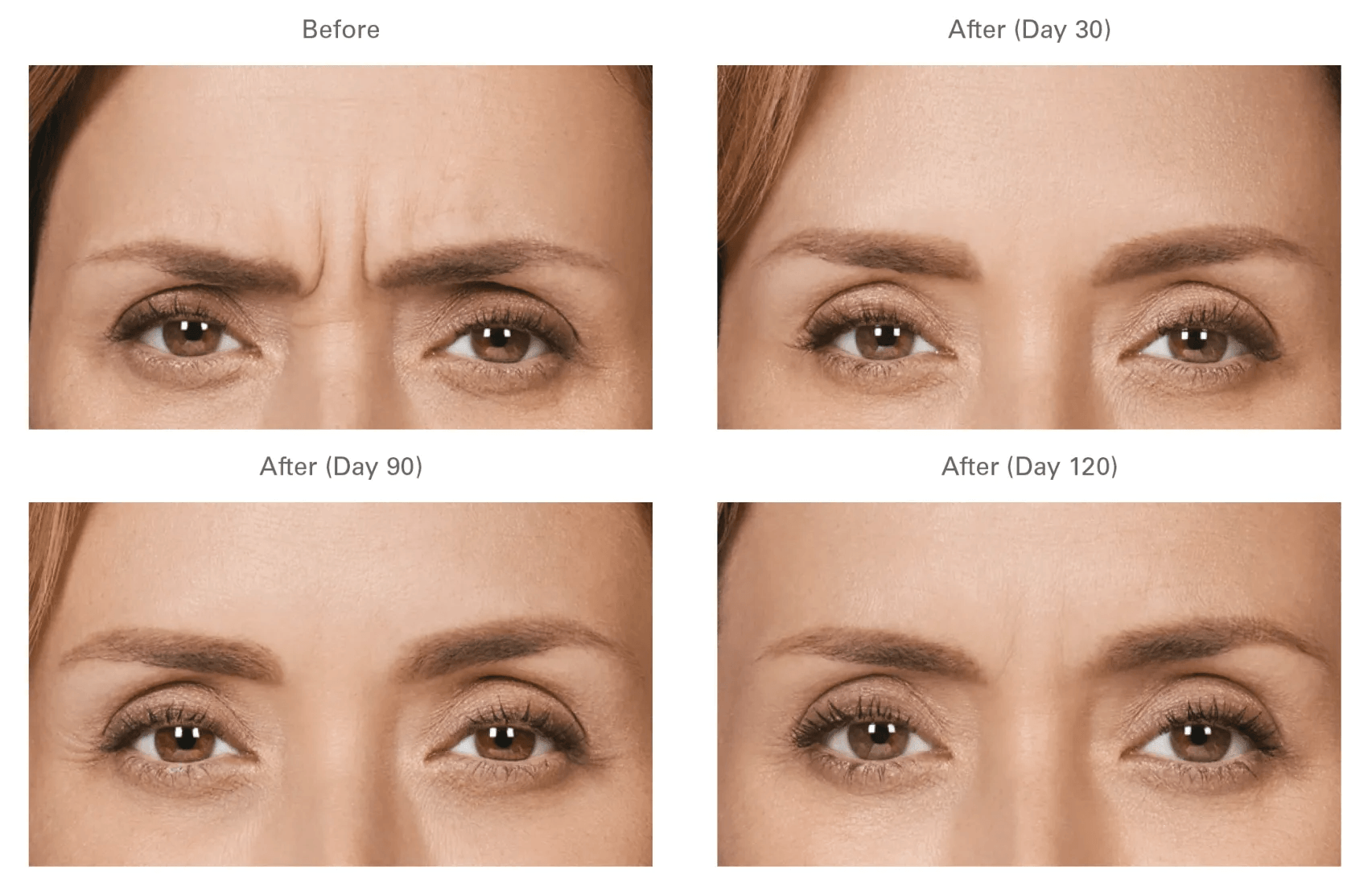
Treatment with BOTOX® Cosmetic takes approximately 10 minutes and requires minimal downtime or recovery—it’s often called a lunchtime procedure.
Think 2 treatments, 1 visit. Ask your doctor aboutreceiving BOTOX® Cosmetic for moderate to severe frown lines and crow’s feet at the same time.
- Important Safety Information
BOTOX® Cosmetic (onabotulinumtoxinA) Important Information
Indications
BOTOX® Cosmetic (onabotulinumtoxinA) is indicated in adult patients for the temporary improvement in the appearance of:
– moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
– moderate to severe lateral canthal lines associated with orbicularis oculi activity
– moderate to severe forehead lines associated with frontalis activity
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX® Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses, including spasticity in children, and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and spasticity and at lower doses.
CONTRAINDICATIONS
BOTOX® Cosmetic is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
WARNINGS AND PRECAUTIONS
Lack of Interchangeability between Botulinum Toxin Products
The potency Units of BOTOX® Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX® Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method.
Spread of Toxin Effect
Please refer to Boxed Warning for Distant Spread of Toxin Effect.
No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), and 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines) have been reported.
Serious Adverse Reactions With Unapproved Use
Serious adverse reactions, including excessive weakness, dysphagia, and aspiration pneumonia, with some adverse reactions associated with fatal outcomes, have been reported in patients who received BOTOX® injections for unapproved uses. In these cases, the adverse reactions were not necessarily related to distant spread of toxin, but may have resulted from the administration of BOTOX® to the site of injection and/or adjacent structures. In several of the cases, patients had pre-existing dysphagia or other significant disabilities. There is insufficient information to identify factors associated with an increased risk for adverse reactions associated with the unapproved uses of BOTOX®. The safety and effectiveness of BOTOX® for unapproved uses have not been established.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of BOTOX® Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Cardiovascular System
There have been reports following administration of BOTOX® of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease. Use caution when administering to patients with pre-existing cardiovascular disease.
Increased Risk of Clinically Significant Effects with Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including generalized muscle weakness, diplopia, ptosis, dysphonia, dysarthria, severe dysphagia, and respiratory compromise from onabotulinumtoxinA (see Warnings and Precautions).
Dysphagia and Breathing Difficulties
Treatment with BOTOX® and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or oropharyngeal muscles that control swallowing or breathing (see Boxed Warning).
Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX® Cosmetic treatment is used in the presence of inflammation at the proposed injection site(s) or when excessive weakness or atrophy is present in the target muscle(s).
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
ADVERSE REACTIONS
The most frequently reported adverse reaction following injection of BOTOX® Cosmetic for glabellar lines was eyelid ptosis (3%).
The most frequently reported adverse reaction following injection of BOTOX® Cosmetic for lateral canthal lines was eyelid edema (1%).
The most frequently reported adverse reactions following injection of BOTOX® Cosmetic for forehead lines with glabellar lines were headache (9%), brow ptosis (2%) and eyelid ptosis (2%).
DRUG INTERACTIONS
Co-administration of BOTOX® Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like compounds) should only be performed with caution as the effect of the toxin may be potentiated. Use of anticholinergic drugs after administration of BOTOX® Cosmetic may potentiate systemic anticholinergic effects.
The effect of administering different botulinum neurotoxin products at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of BOTOX® Cosmetic.
USE IN SPECIFIC POPULATIONS
There are no studies or adequate data from postmarketing surveillance on the developmental risk associated with use of BOTOX® Cosmetic in pregnant women. There are no data on the presence of BOTOX® Cosmetic in human or animal milk, the effects on the breastfed child, or the effects on milk production.
(accordion cont.)
BOTOX & FILLERS: PRE-TREATMENT INSTRUCTIONS
• Do not consume alcoholic beverages at least 24 hours prior to treatment (alcohol may thin the blood and increase the risk of bruising).
• Avoid anti-inflammatory/blood thinning medications, if possible, for a period of two weeks before treatment. Medications and supplements, Ibuprofen, Motrin, Advil, Aleve and other NSAIDs have a blood thinning effect and can increase the risk of bruising and swelling after injections.
• Schedule your Dermal Filler and Botox appointment at least 2 weeks prior to a special event which you may be attending, such as a wedding or a vacation. Results from Botox injections will take approximately 4 to 7 days to appear. Also bruising and swelling may be apparent at that time period.
• Discontinue Retin-A 2 days before and 2 days after treatment.
• Reschedule your appointment at least 24 hours in advance if you have a rash, cold sore or blemish on the area to be treated.
• Be sure to have a good breakfast, including food and drink before your procedure. This will decrease the chances of lightheadedness during your treatment.
• You are not a candidate for injectables if you are pregnant or breastfeeding.
BOTOX: POST-TREATMENT INSTRUCTIONS
• Do not manipulate the treated area for 3 hours following treatment.
• Do not receive facial/laser treatments or microdermabrasion after Botox injections for at least 10 days. Ask Dr. Reidy if you are not sure about the time frame for certain services.
• Avoid consuming alcohol for 8 hours after treatment.
• Do not lie down for 4 hours after your Botox treatment. This will prevent the Botox from tracking into the orbit of your eye and causing drooping eyelid.
• It can take approximately 4 to 7 days for results to be seen. If the desired result is not seen after 2 weeks of your treatment you may need additional Botox. You are charged for the amount of product used. Therefore, you will be charged for any product used during any touch-up or subsequent appointments.
• Do not perform activities involving straining, heavy lifting or vigorous exercise for 6 hours after treatment. This will keep the Botox in the injected area and not elsewhere.
FILLERS: POST-TREATMENT INSTRUCTIONS
• Avoid significant movement or massage of the treated area, unless instructed to do so by Dr. Reidy.
• Avoid strenuous exercise for 24 hours.
• Avoid extensive sun or heat for 72 hours.
• Avoid consuming alcohol for 8 hours after treatment.
• If you have swelling you may apply a cool compress for 15 minutes each hour. You may use the ice pack we provide as much as is comfortable for the first 1-2 hours after treatment.
• Use Tylenol for discomfort.
• Try to sleep face up and slightly elevated if you experience swelling.
JUVÉDERM® Collection FAQs & Safety Information
- Which areas can be treated with JUVÉDERM®?
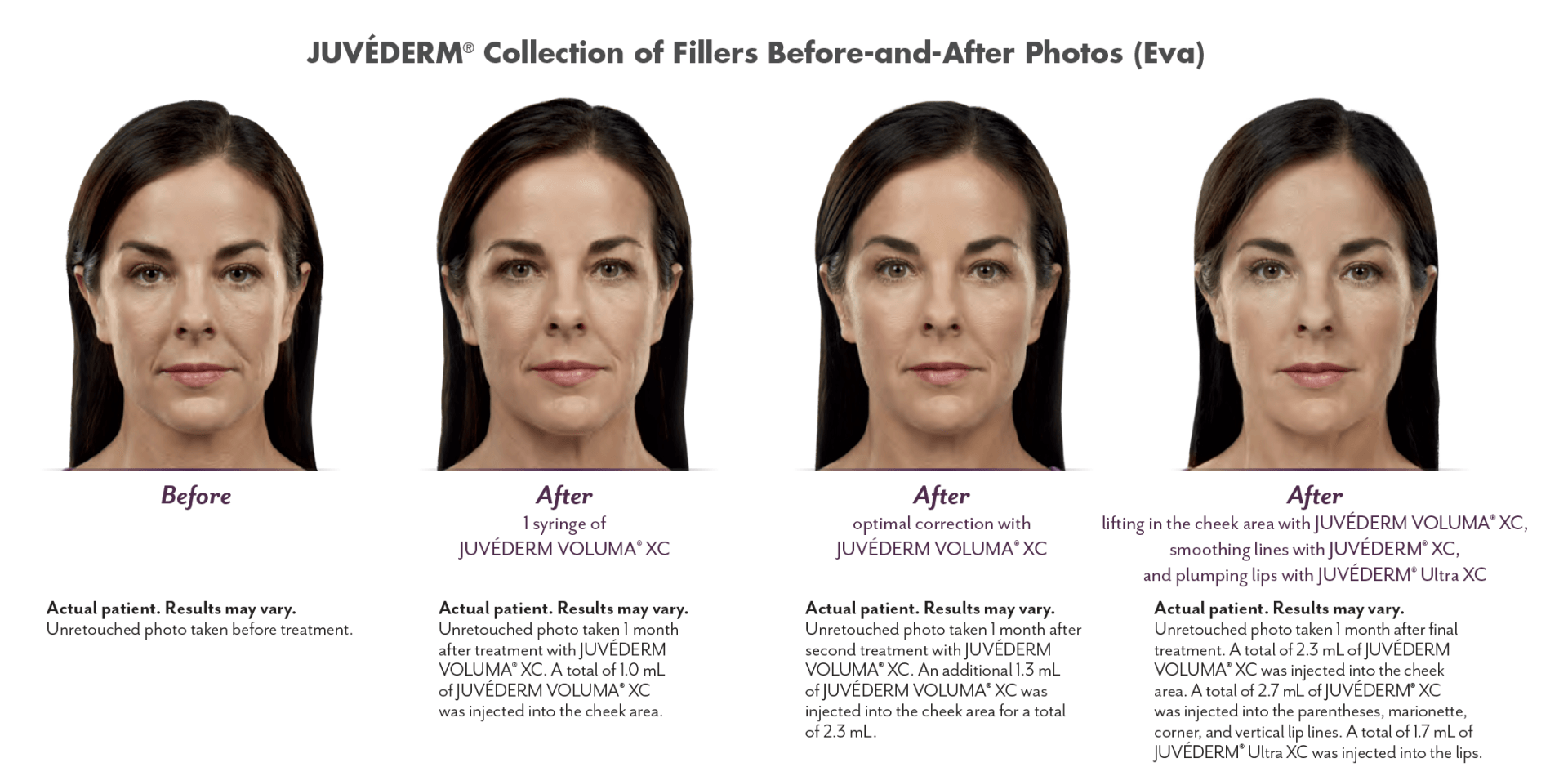
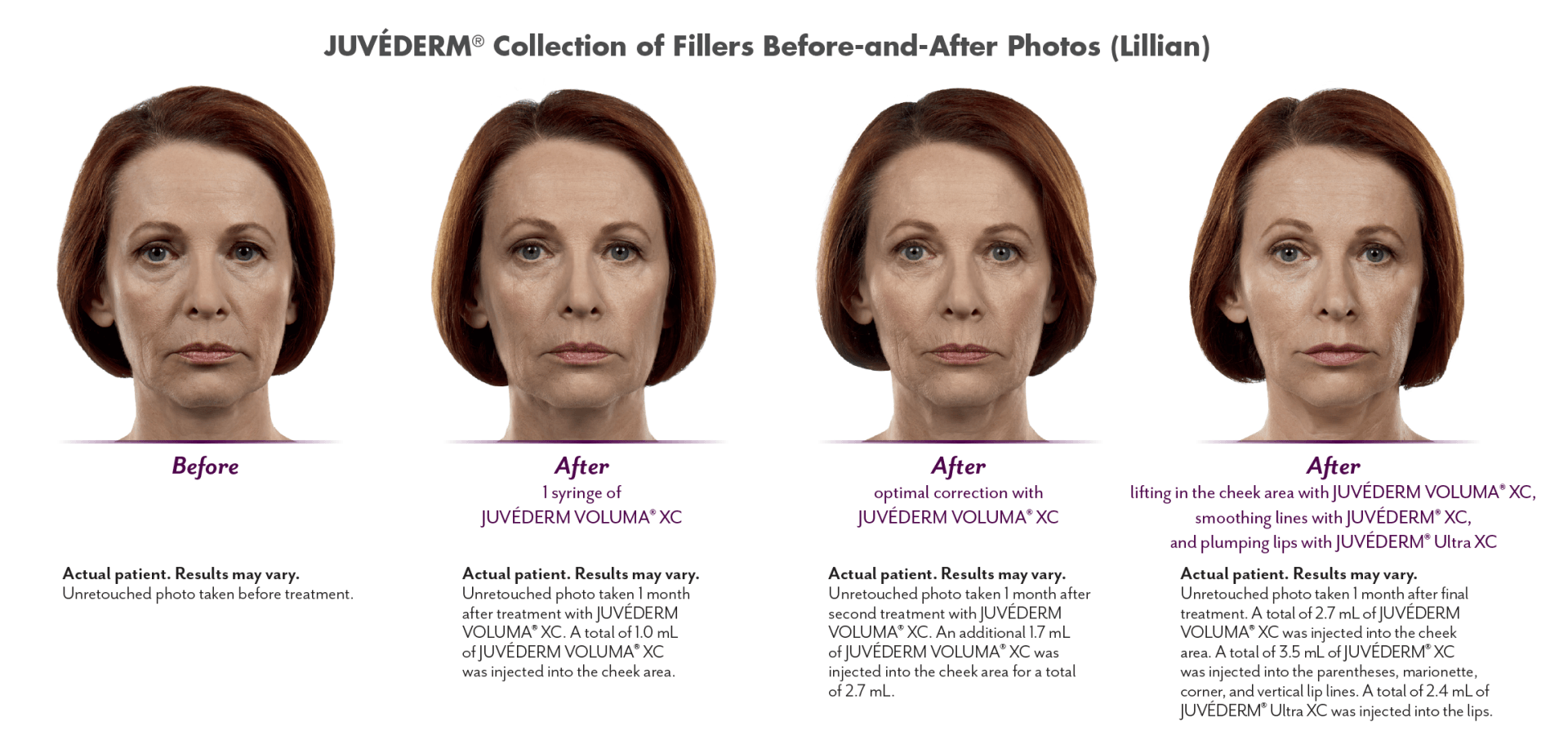
The JUVÉDERM® range of fillers can correct age-related loss in the cheeks, lips, lip lines, and other facial wrinkles and folds in individuals over the age of 21.
- Why should I choose JUVÉDERM®?
JUVÉDERM® is the world's #1 selling dermal filler collection, and with good reason! JUVÉDERM® is FDA-Approved, so you can rest assured that it is safe. Using JUVÉDERM® offers:
- Instant results
- Nonsurgical treatments
- Natural-looking, long-lasting results
- In-office procedures
- Important Safety Information
JUVÉDERM® Collection of Fillers Important Information
INDICATIONS
JUVÉDERM VOLUMA® XC injectable gel is indicated for deep (subcutaneous and/or supraperiosteal) injection for cheek augmentation to correct age-related volume deficit in the mid-face in adults over the age of 21.
JUVÉDERM® Ultra XC and JUVÉDERM® Ultra Plus XC injectable gels are indicated for injection into the mid-to-deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds).
JUVÉDERM VOLLURE™ XC injectable gel is indicated for injection into the mid-to-deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds) in adults over the age of 21.
JUVÉDERM® Ultra XC injectable gel is indicated for injection into the lips and perioral area for lip augmentation in adults over the age of 21.
JUVÉDERM VOLBELLA® XC injectable gel is indicated for injection into the lips for lip augmentation and for correction of perioral rhytids in adults over the age of 21.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
These products should not be used in patients who have severe allergies, marked by a history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with a history of allergies to Gram-positive bacterial proteins or lidocaine contained in these products.
WARNINGS
Do not inject into blood vessels. Introduction of these products into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Take extra care when injecting soft-tissue fillers; for example, inject the product slowly and apply the least amount of pressure necessary. Rare, but serious, adverse events associated with the intravascular injection of soft-tissue fillers in the face have been reported and include temporary or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis, and damage to underlying facial structures. Immediately stop the injection if a patient exhibits any of the following symptoms: changes in vision, signs of a stroke, blanching of the skin, unusual pain during or shortly after the procedure. Patients should receive prompt medical attention and, possibly, evaluation by an appropriate healthcare professional specialist should an intravascular injection occur
Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled
PRECAUTIONS
In order to minimize the risk of potential complications, these products should only be used by healthcare professionals who have appropriate training, experience, and knowledge of facial anatomy
Healthcare professionals are encouraged to discuss the potential risks of soft-tissue injections with their patients prior to treatment and ensure that patients are aware of signs and symptoms of potential complications
The safety and effectiveness for the treatment of anatomic regions other than the mid-face with JUVÉDERM VOLUMA® XC; facial wrinkles and folds with JUVÉDERM® Ultra XC, JUVÉDERM® Ultra Plus XC, and JUVÉDERM VOLLURE™ XC; and the lips and perioral area with JUVÉDERM® Ultra XC and JUVÉDERM VOLBELLA® XC have not been established in controlled clinical studies
As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Follow standard precautions associated with injectable materials
The safety for use during pregnancy, in breastfeeding females, and in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied
The safety for use of JUVÉDERM VOLUMA® XC in patients under 35 or over 65 years, JUVÉDERM® Ultra XC and JUVÉDERM® Ultra Plus XC in patients under 18 years, and JUVÉDERM VOLLURE™ XC and JUVÉDERM VOLBELLA® XC in patients under 22 years has not been established
Use with caution in patients on immunosuppressive therapy
Patients who are using products that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may experience increased bruising or bleeding at treatment sites
If laser treatment, chemical peel, or any other procedure based on active dermal response is considered after treatment, or if these products are administered before the skin has healed completely, there is a possible risk of an inflammatory reaction at the treatment site
Patients who experience skin injury near the site of implantation may be at a higher risk for adverse events
The safety of JUVÉDERM VOLUMA® XC injectable gel for use in patients with very thin skin in the mid-face has not been established
Patients may experience late onset nodules with use of dermal fillers, including JUVÉDERM VOLUMA® XC
Patients may experience late onset adverse events with use of dermal fillers
ADVERSE EVENTS
The most commonly reported side effects for JUVÉDERM® injectable gels were injection-site redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. For JUVÉDERM VOLBELLA® XC, dryness was also reported. For JUVÉDERM VOLUMA® XC, side effects were predominantly moderate in severity, with duration of 2 to 4 weeks; for JUVÉDERM® Ultra XC, JUVÉDERM® Ultra Plus XC, or JUVÉDERM VOLLURE™ XC, they were mostly mild or moderate in severity, with duration of 14 days or less; and for JUVÉDERM VOLBELLA® XC, they were predominantly mild or moderate, with duration of 30 days or less.
To report an adverse reaction with any product in the JUVÉDERM® Collection, please call Allergan at 1-800-433-8871. Please visit JuvedermDFU.com for more information.
Products in the JUVÉDERM® Collection are available by prescription only.
BOTOX® & Fillers: Pre- and Post-Treatment Instructions
- BOTOX® & Fillers Pre-Treatment Instructions
- Do not consume alcoholic beverages at least 24 hours prior to treatment (alcohol may thin the blood and increase the risk of bruising).
- Avoid anti-inflammatory/blood thinning medications, if possible, for a period of two weeks before treatment. Medications and supplements, Ibuprofen, Motrin, Advil, Aleve and other NSAIDs have a blood thinning effect and can increase the risk of bruising and swelling after injections.
- Schedule your Dermal Filler and Botox appointment at least 2 weeks prior to a special event which you may be attending, such as a wedding or a vacation. Results from Botox injections will take approximately 4 to 7 days to appear. Also bruising and swelling may be apparent at that time period.
- Discontinue Retin-A 2 days before and 2 days after treatment.
- Reschedule your appointment at least 24 hours in advance if you have a rash, cold sore or blemish on the area to be treated.
- Be sure to have a good breakfast, including food and drink before your procedure. This will decrease the chances of lightheadedness during your treatment.
- You are not a candidate for injectables if you are pregnant or breastfeeding.
- BOTOX® Post-Treatment Instructions
- Do not manipulate the treated area for 3 hours following treatment.
- Do not receive facial/laser treatments or microdermabrasion after Botox injections for at least 10 days. Ask Dr. Reidy if you are not sure about the time frame for certain services.
- Avoid consuming alcohol for 8 hours after treatment.
- Do not lie down for 4 hours after your Botox treatment. This will prevent the Botox from tracking into the orbit of your eye and causing drooping eyelid.
- It can take approximately 4 to 7 days for results to be seen. If the desired result is not seen after 2 weeks of your treatment you may need additional Botox. You are charged for the amount of product used. Therefore, you will be charged for any product used during any touch-up or subsequent appointments.
- Do not perform activities involving straining, heavy lifting or vigorous exercise for 6 hours after treatment. This will keep the Botox in the injected area and not elsewhere.
- Fillers Post-Treatment Instructions
- Avoid significant movement or massage of the treated area, unless instructed to do so by Dr. Reidy.
- Avoid strenuous exercise for 24 hours.
- Avoid extensive sun or heat for 72 hours.
- Avoid consuming alcohol for 8 hours after treatment.
- If you have swelling you may apply a cool compress for 15 minutes each hour. You may use the ice pack we provide as much as is comfortable for the first 1-2 hours after treatment.
- Use Tylenol for discomfort.
- Try to sleep face up and slightly elevated if you experience swelling.
Ready to schedule an appointment?
Ready to schedule a consultation, or need to ask questions about our Aesthetics or Wellness services? You can reach our Aesthetics Coordinator by calling our office at (256) 759-9269 and dialing extension 107.
Follow Us
-
P R O G R E S S 💙 We are quite literally OBSESSSSSED with these results of this beautiful patient of ours. These pictures are SIX months apart!! This sweet patient came to us last year needing guidance on how to naturally improve the overall integrity of her skin. After reaching her goal of losing 50 pounds, her skin on the face and neck became lax, which bothered her. She also wanted to improve fine lines + wrinkles, her under eyes, and overall texture of the skin. Our Medical Aesthetician, Alex, developed a protocol for her that fit her lifestyle + goals and we are blown away! What we have done 👇 - custom ZO protocol - Morpheus8 full face + neck What we’re planning for 👇 - more Morpheus8 treatments - Continuing ZO protocol Remember, achieving skin health is not one size fits all. If you’re ready to start your journey, let’s talk! natural skincare | ZO SkinHealth | routines #women4womenobgyn #naturalaesthetics #medspa #morpheus8 #microneedling #ZOskinhealth #skincare #huntsville #ihearthsv #sohsv #rocketcity #skinresults #skintipsButton
-
The T H R E E Pillars of WELLNESS 🩵 Our Wellness House is right around the corner + we are so excited for this years event! Over the next couple of weeks leading up to our Wellness Open House, we will be sharing the 3 main categories of Wellness 😍 Wellness is an integrative process of PHYSICAL, EMOTIONAL, and SOCIAL dimensions that impact an individuals overall health. PHYSICAL HEALTH 👉 being mindful about how you fuel + take care of your body. EMOTIONAL HEALTH 👉 your ability to cope with positive + negative emotions. SOCIAL HEALTH 👉 relationships we have + how we interact with others. At our Wellness Open House, you will have the opportunity to hear from a variety of community experts on these 3 Pillars of Wellness; Physical, Emotional, and Social 🩵 We hope to see you on Thursday, April 11th from 5:00 p.m. - 7:00 p.m. for our Wellness Open House!Button
-
Say hello to our new new 💙 COMPLEXION CLARIFYING SERUM ✨ Engineered with ZO sebum-targeting science, Complexion Clarifying Serum is clinically proven to reduce surface oil, congestion, and visible redness for continually clear + balanced complexion. WHY WE LOVE IT ⬇️ - proven to decongest the skin + reduce surface shine in 2 weeks - Minimizes excess sebum with daily use, while providing optimal hydration for a range of skin types - Reduces redness for a more uniform complexion - Light weight, layering easily with your other ZO products DO I NEED THIS ⬇️ - We are loving complexion clarifying serum for our friends with acne + melasma, and redness 🩵 Ready to add this to your protocol? DM us for how to get started 💙 skincare | skincare tips | ZO SkinHealth #huntsville #ihearthsv #sohsv #hellohuntsville #nashville #hazelgreenal #madisonal #cullmanal #fayetvilletn #medicalgradeskincare #injectables #laserButton
-
We are just T H R E E weeks away from our Wellness Open House! 🙌 Today, we're breaking down what Wellness actually IS 👇 Wellness is the quality or state of being in good health especially as an actively sought goal. Individuals who practice Wellness are practicing healthy habits on a daily basis to attain better physical and mental health outcomes, so that instead of just surviving, you are THRIVING. There are areas of lifestyle that are dimensions of overall wellness, including: 🩵 social connection 🩵 exercise 🩵 nutrition 🩵 sleep 🩵 mindfulness At our Wellness Open House, we are excited to dive into each of these areas of Wellness with some of the best Wellness Practitioners in the Huntsville area! Our Wellness Open House is hosted at Women4Women OBGYN on Thursday, April 11th. Doors open at 5:00 p.m.! Small bites + beverages + lots of learning! Link in bio to register and let us know you're coming! Registration is not required, but we'd love to get a head count. Aesthetics | Wellness | Pure Barre | Chiropractic Care | Acupuncture | Yoga | Medical Weight Loss | natural skincare | nutrition | wellbeing #huntsvilleal #sohsv #hellohsv #WeareHSV #rocketcity #MadisonAL #owenscrossroadsal #northalabama #cullmanalabama #Fayetteville #hazelgreenalButton
-
Sharing O U R results 😍 Our very own Esti Bestie (our medical Aesthetician) Alex, and her results after a custom skincare protocol + in office treatments 🤍 Alex’s main skin concerns are ruptured capillaries, unwanted redness and hyperpigmentation. We curated a protocol for her and are OBSESSSSSSED with her results so far. The enhancement in her complexion is SUPREME 💙 ⁃ pictures are 8 weeks apart ⁃ Custom skincare protocol | ZO | SkinMedica ⁃ In office treatments Let’s help you achieve your best skin yet! Our consult appointments are FREE! DM us to get started 🥰 #huntsville #madisonal #owenscrossroadsal #hazelgreenal #athensal #fayetvilletn #nashville #birminghamal #cullmanal #skincare #facail #medspaButton
-
Welcome to the AESTHETIC C O N S U L T A T I O N 👋 The step taken to start your journey + achieve your goal(s). Our consults are specially designed for us to get to know you and more importantly, for you to get to know us. We provide you with all our knowledge and guide you to the best place to start. To us, relationships are everything and we can’t wait to start one with YOU 🫶 Whatever you’re looking for, we got you covered (and consults are FREE) 🙌 Our DMs are open! Start your journey today 👇 📲 256-759-9269 👩💻 women4womenhsv.com #Women4WomenOBGYN #womeninmedicine #aesthetics #wellness #ZOSkinHealth #SkinMedica #medicalgradeskincare #botox #fillers #injectables #microneedling #PRP #laser #IPL #morpheus8 #huntsvilleal #sohsv #hellohsv #weareHuntsville #madisonal #owenscrossroadsAL #nashville #birminghamalButton
-
R E S U L T S 🫶 We are loving these results after just TWO treatments with our beloved Lumecca IPL👏🏻 💡IPL, or intense pulsed light, targets PIGMENT (specifically redness, vascular lesions, sun damage, and age spots). In just 3 treatments, we can get exceptional clearance of any unwanted discoloration in the skin. 🏃🏼♀️Hurry, though! We stop IPL treatments in April! We do not pursue IPL treatments with our patients in the summer because we cannot treat over tanned skin. Treating over tanned skin does not give an authentic result. We're here for long-term, authentic, and lasting results 🩵 Consults are FREE! Call to book! #women4womenobgyn #IPL #photofacial #clearskin #huntsvilleal #ihearthsv #results #skincare #skincarelovers #hellohuntsville #WeareHuntsville #nashvilletn #birminghamal #fayetvilletn #owenscrossroadsal #madisonal #ZOSkinHealth #SkinMedicaButton
-
📅 MARK YOUR CALENDARS! Join us for our 2nd annual Wellness Open House hosted at Women4Women OBGYN! Guests will have the opportunity to learn from the leading wellness experts in the Huntsville area on physical, mental, and emotional wellbeing. From holistic medicine, midwifery, physical health, medically managing weight loss, nutrition, and MORE, guests will have the opportunity to connect with the best Wellness Practitioners in the Huntsville area 🥳 THE DETAILS 👇 📅 Thursday, April 11th from 5:00 - 7:00 p.m. 📍 Women4Women OBGYN 🙋🏼♀️ Let us know you're coming by registering with the link in our bio 👏🏻 STAY TUNED for details on our exclusive promotions, raffles, who is attending and MORE! We are so excited for you to join us for night of connecting + learning more ways to manage your physical, mental, and emotional health. Questions?! Our DMs are open! See you soon 👋 #women4womenobgyn #womeninmedicine #Wellness #Aesthetics #huntsvilleevent #holisticcare #holistichealth #events #ihearthsv #weareHuntsville #weightloss #madisonal #owenscrossroadsal #fayetvilletn #zoskinhealth #skinmedicaButton
-
Routine | noun | a sequence of actions regularly followed; a fixed program. Having a routine, morning + night, sets your day up for success and helps you to wind down at night. Routines improve sleep quality, decrease anxiety, and impact overall health + wellness. We’re here for the evening skin routines - simple, yet effective, helping us wind down as we wipe our day away 🩵 What are some of your favorite routines? #routine #hellohuntsville #sohsv #ihearthsv #skincare #madisonal #owenscrossroadsal #rocketcity #wellness #skinhealth #skingoalsButton
-
Happy International Women's Day! 💓 May we honor the resilience, achievements, and advancements of women worldwide. Let us continue to empower each other and generations to come as we work towards equality for ALL women. We love to hear from YOU. Tag a woman in your life who has made an impact on you. #internationalwomensday #womensrights #feminism #womenownedbusiness #women4womenobgyn #womeninmedicine #womensequality #HuntsvilleAL #ihearthsv #sohsv #hellohsvButton
-
RESULTS 👏🏻 Before and after TWO treatments with Microneedling + custom ZO protocol 🩵 This patient has been working with our Aesthetics Team for several years. Starting out with injectables to correct fine lines and wrinkles, turned into a multi-modality treatment plan in order to correct hyperpigmentation + mild redness in order to restore SKIN HEALTH. What we've done 👇🏻 - Custom ZO protocol - 3 sessions of Microneedling - quarterly Tox - chemical peels as needed (seasonly dependent) What we're planning for 👇 - quarterly microneedling - quarterly IPL treatment Achieving SKIN HEALTH looks different for each patient and we're here for that! DM us to get started with your FREE consultation. 👩💻 women4womenhsv.com 📲 256-759-9269 #women4womenobgyn #womeninmedicine #skincare #ZOSkinHealth #SkinHealth #Healthyskin #glowingskin #huntsvilleal #madisonal #nashvilletn #aesthetics #wellness #medspa #botox #filler #microneedling #skincareroutineButton
-
To commemorate Women’s History Month, we at Women4Women pledge to walk 31 miles in 31 days in honor of influential women. Follow our journey this month to discover whom we are celebrating and our progress towards our goal. Join us and share your own journey!Button
-
Dr. Reidy spent some time with WAAY 31 News yesterday discussing the Supreme Court ruling regarding frozen embryos. We at W4W are concerned about the possibility that this ruling could have far-ranging impacts on women’s health. https://www.waaytv.com/news/huntsville-obgyn-weighs-in-on-impact-of-supreme-courts-ivf-ruling/article_155e5efc-d6bf-11ee-8996-1b25a6e8ed84.htmlButton
-
Our ZO GLOW Event is less than O N E month away! Today, we're answering all your questions on our signature ZO Glow Service ⚡️ Our Signature ZO GLOW service is also known as our signature facial. During our Signature ZO service, we use a serum called the "Stimulator Peel." The Stimulator Peel is a no downtime, no peel, peel that is safe for all patients and all skin types. During this service, we are able to stimulate cell turnover to treat skin conditions and concerns such as 👇 ⚡️ acne ⚡️ fine lines + wrinkles ⚡️ discoloration ⚡️ uneven skin tone ⚡️ texture You will leave with a GLOW like no other 👏🏻 Be sure to call to secure your spot for our ZO GLOW event! By appointment only on March 22nd and 23rd. Your Signature ZO Glow service is $150 and we MATCHING you $150 in ZO Product. Don't miss out! 📲 256-759-9269 👩💻 women4womenhsv.com #women4womenhsv #ZOGlow #ZOSkinHealth #Event #aesthetics #wellness #facial #service #huntsvilleal #madisonal #owenscrossroadsal #aesthetician #sohsv #hellohsv #huntsvillealButton
-
MARK YOUR CALENDARS + JOIN US ✨ We have an exciting ZO Glow Event planned just for YOU 🎉 We couldn't think of a better way to rejuvenate after Spring Break than with an exclusive ZO SkinHealth Event. Grab a friend + and let's G L O W! Come in and receive our signature ZO Glow Service for $150 and we will M A T C H you $150 in ZO SkinHealth product! The details 👇 📅 Thursday, March 21st from 10:00-5:00 👉 by appointment only 📅 Friday March 22nd from 10:00 - 1:00 👉 by appointment only 📍 Women4Women OBGYN; 320 Pelham Avenue Suite 301 📲 TO BOOK: 256-759-9269 🙋🏼♀️ A card is REQUIRED to be on file to book your appointment. If you no call no show, or do not provide a 24 hours notice of cancellation, you will be charged a $75 fee. Your $150 product match MUST be used at the time of your Glow appointment. If you do not use it, you will lose it. Small bites + beverages + fun! We can't wait to GLOW with you! #event #women4womenobgyn #ZOGlow #ZOskinhealth #huntsvilleal #ihearthsv #sohsv #hellohsvButton
-
At Women4Women OBGYN, we stand by the recent statement from The Medical Association of Alabama expressing extreme concern over the recent Alabama Supreme Court ruling regarding embryos as children. We are deeply saddened for the families who are impacted by this decision. For a lot of families, IVF is the ONLY option to have children. Medical Association of Alabama article is LINKED in our bio + stories. Join us in advocating for reproductive health care in Alabama. #ivf #alabama #ivfalabama #women4womenobgynButton
-
TONING 🤍 The 2nd step in your skincare routine after cleansing + generally everyone’s favorite step! By toning the skin, we can PREPARE the skin for subsequent products in your routine. We have THREE toners with ZO SkinHealth + we’ll start with everyone’s favorite 👇 Complexion Renewal Pads — for NORMAL to OILY skin. The Complexion Renewal pads help to minimize surface oil, decongest pores, prevent breakouts, replenish hydration while also providing anti-oxidant and anti-irritant benefits. Oil Control Pads — ACNE TREATMENT! For OILY to ACNE-PRONE skin. If you struggle with cystic + hormonal acne breakouts, these are for you! 2% salicylic acid provides maximum strength acne solution, while minimizing surface oil. Calming Toner — for DRY, SENSITIZED skin. Calming toner balances pH to RESTORE skin health. Hydrtes + calms skin and subtly exfoliates dry, dull skin. A HOT Tip — use calming toner BEFORE complexion renewal pads or the oil control pads to prepare the skin for your pads. We also love to use the Oil Control Pads on the nose to remove blackheads! #women4womenobgyn #ZOSkinHealth #toners #complexionrenewalpads #oilcontrolpads #calmingtoner #huntsvilleal #ihearthsv #sohsv #hellohuntsville #acnetreatment #skincareishealthcareButton
-
Ingredients your skin will love 💓 Curious to find an ingredient your skin will love? DM us for a free consult appointment! #skincarelove #glowingskin #skinhealth #womeninmedicine #aesthetics #wellness #ZOSkinHealth #SkinMedica #skincareingredients #huntsville #ihearthsv #hellohsv #WeAreHuntsvilleButton
-
Happy Valentine's Day! We LOVE ya'll! 💓 #valentinesday #love #women4womenobgynButton
-
It's all COOL! ❄️ FDA-cleared, safe and effective to target + eliminate stubborn fat in 9 areas of the body 😲 Curious to see if CoolSculpting is right for you? Your free consult is one phone call away 🩵 📲 256-759-9269 👩💻 women4womenhsv.com #women4womenobgyn #coolsculpting #womeninmedicine #aesthetics #wellness #midwifery #obgyn #fatreduction #fatfreezingtechnologButton
-
UBERLUBE 💓 The most luxe lubricant (if you know, you know 😉) Smooth, silky, and body friendly. Uberlube does not harbor bacteria + yeast + mold. Shop using the link in our bio or pop in our office to purchase! #uberlube #womenshealth #ihearthsv #sohsv #valentinesday #wearehuntsvilleButton
-
Some skincare matchups we love 💓 What's your favorite match? #women4womenhsv #sohsv #skincare #zoskinhealth #healthyskin #aesthetics #wellness #takingcareofwomenButton
-
Dr. Rose reporting for duty! 💗👩⚕️ How precious is Dr. Reidy’s granddaughter, Rose, measuring the belly of her baby? Now, will she will follow in her mom’s footsteps and be an Anesthesiologist or follow in her Nona’s footsteps and be an OB/GYN? We are Team Nona 😉 #thefutureisfemale #empoweringyoungminds #futuredoctor #huntsville #hellohuntsville #sohsv #ihearthsvButton
-
Send this to your significant other to let them know what you really want for Valentine’s Day 💕 Also, this post DiamondGlow GLOW is what we’re really in love with 😍Button
-
MonaLisa Touch 🩵 A fractional CO2 laser that treats vaginal dryness, pain associated with intercourse, and mild urinary incontinence. When all other treatment options have failed, or if you aren't seeing the success that you desire, MonaLisa can help you! What we recommend 👇 🩵 start with a consultation appointment to see if MonaLisa is the right treatment option for you based on your goals 🩵 3 treatments for optimal results. The laser uses a stacking technology, so your maximum benefit is after the 3rd treatment. Don't be afraid to talk about it. We are here for you! 👇 👩💻 women4womenhsv.com 📲 256-759-9269 #women4womenobgyn #monalisatouch #vaginaldryness #painwithintercourse #menopause #perimenopause #ihearthsv #helpingwomen #takingcareofwomen #womeninmedicine #womenshealth #obgyn #doctorsofinstagram #obstetrics #gynecologyButton
-
I P L 💡 intense ⚡️pulsed ⚡️light WHAT IS IT 👇 IPL (intense pulsed light) is a light treatment that targets redness, vascular lesions, and dark spots in the skin. IPL improves the overall clarity of skin; making the skin more even toned with each treatment. DO I NEED IT 👇 If you have unwanted pigment in your skin, or just need a refresh to your complexion, then IPL could be a good option for you! 30 minute treatment | NO DOWNTIME | FREE consultation Clear skin ahead 👇 📲 256-759-9269 💻 women4womenhsv.com #women4womenobgyn #IPL #photofacial #hsvskin #skinconsult #skincare #hellohuntsville #wearehsv #rocketcity #madisonal #medspa #aestheticsButton
-
👩⚕️Happy National Female Physicians Day to these two super stars! 🌟 We are grateful for Dr. Reidy and Dr. Alexander and the work they do day in and day out not only for their patients, but for Women's Health in North Alabama. They make a remarkable impact on the lives of many, many women; improving their quality of life, as they journey with them through every season of womanhood. Please join us in celebrating them today! #nationalfemalephysiciansday #womeninmedicine #women4womenobgyn #womenshealthcare #obstetrics #gynecology #womenhelpingwomen #obgyn #aesthetics #wellness #midwifery #huntsville #ihearthsv #rocketcityButton
-
Our February Promotions are live + we are in L O V E 💓 Here's what we have in store this month 👇 💓 EYE love you 👉 a box of UpNeeq, 1 SkinMedica Instant Bright Eye Cream, and 1 ZO SKinHealth Growth Factor Eye Serum 💓 LOVE your glow 👉 Combine DiamondGlow + IPL this month to achieve your clearest skin yet! 💓 Love to be cool 👉 purchase 6 cycles of CoolSculpting and we'll throw in 2 for FREE! 💓 GIFT with Purchase 👉 for every $250 spent on ZO, we'll give you a travel size Daily Power Defense and 3 skin brightening sheet masks for FREE! Which promotion are you falling for this month? #women4womenhsv #February #lovemonth #lovelythings #sohsv #huntsvilleal #ihearthsv #sohsv #womeninmedicine #obgyn #medspa #Midwifery #coolsculpting #ZOskinhealth #IPL #lumecca #diamondglowButton
-
Self-love reminders to kick off love month 💓 Do you have any daily affirmations you speak over yourself? #love #lover #selflove #selfcare #selfcareisnotselfish #affirmations #february #women4womenhsv #women4womenobgyn #takingcareofwomenButton
-
W E L L N E S S WEDNESDAY <3 Welcome to Wellness Wednesday with our Women4Women Team! Wellness is a pillar of our practice vision at Women4Women OBGYN. We believe in comprehensive medical care for women by women. Comprehensively, we include Midwifery, Aesthetics, and Wellness services. Wellness is the active pursuit of activities, choices, and lifestyles that lead to a state of holistic health. Wellness includes a great amount of self-responsibility. Individually, we have power over our choices. Wellness is an active process, rather than a static state. To kick off our series, we are starting with finding BALANCE in our lives. We asked several of our staff members how they find balance in their life and we think you will love some of their responses. How do you find Balance in your life?! We’d love to hear below! #wellness #livingwell #balance #findingbalance #ihearthsv #sohsv #hellohsv #huntsvilleal #rocketcity #holistichealth #holisticmedicine #aesthetics #midwifery #women4womenobgyn #reels #reelideas #viralButton
-
NEXPLANON VS. IUDS 👇 Deciding between a Nexplanon and an IUD can be tricky! Both birth control devices have similarities but also major differences. Today, we're chatting about the differences between IUD and Nexplanon 👇 🩵 Nexplanon is a small, flexible implant that is placed in the ARM. 🩵 IUDS are small, T-shaped devices that are placed in the UTERUS. 🩵 Both provide long term birth control without having to worry about taking a pill daily. Nexplanon provides 3 YEARS of pregnancy prevention. IUDs (depending on brand) can last up to 8 YEARS. 🩵 Both can be inserted in your OB/GYNs office. 🩵 Both can affect bleeding patterns. Mirena IUD can actually be used as a treatment for heavy bleeding for up to 5 years. Be sure to talk to your healthcare provider if you're having a hard time deciding which method of birth control is right for you. 📲 256-759-9269 👩💻 women4womenhsv.com #IUDs #IUDvsNexplanon #Nexplanon #birthcontrol #womenshealth #obgyn #gynecology #obstetrics #womeninmedicine #doctorsofinstagram #takingcareofwomen #rocketcity #wearehuntsville #ihearthsv #sohsvButton
-
C U S T O M I Z A B L E 🫶 One size certainly does not fit all, especially when it comes to your skin. Everyone's skin has different sensitivities + needs and we understand that more than anyone. The perfect part about the DiamondGlow Facial is that it is 100% customizable to your skin. As you age, or go through hormonal changes such as a pregnancy, your skin will change too. The ability for the DiamondGlow to adapt to these changes is truly incredible + further proof that the DiamondGlow can take care of your skin during every stage. Ready to try our most popular facial? Call our office to check our availability 👇 📱256-759-9269 #Women4WomenOBGYN #skincare #DiamondGlow #DiamondTip #AllerganAesthetis #Botox #Filler #skincareinpregnancy #huntsvilleskin #ihearthsv #sohsv #hellohsv #rocketcity #skincarelovers #skincareguru #nashvilleskin #fayetvilleskinButton
-
Your comfort is OUR priority 🫶 We understand that sometimes doctors appointments can bring anxiety, especially when you may have an in-office procedure performed. Meet Polly the ProNox 👇 We are proud to offer our patients ProNox (laughing gas) upon request for certain in office procedures. If you're coming in for procedures such as IUD insertion, biopsies, or colposcopies you can use laughing gas in our office. Polly the ProNox (we love to give everything a name around here) will help to decrease anxiety which can help ease pain during certain procedures. Our team is committed to making your experience as comfortable as possible, and Polly the ProNox certainly helps us achieve that! 🌟Polly the ProNox is a $50 service fee 🌟 #patientcomfort #laughinggas #ease #doctorsoffice #gynecology #obstetrics #midwifery #IUDinsertion #mirena #kyleena #inofficeproceudre #comfortiskey #ihearthsv #huntsvillealButton
-
What people are S A Y I N G 🫶 We are so grateful for the opportunity to serve each patient that walks through our clinic doors with respect + care + kindness. To learn more about our Provider Team or to schedule an appointment 👇 💻 women4womenhsv.com 📱256-759-9269 #women4womenhsv #women4women #ihearthsv #sohsv #hellohsv #rocketcity #obgyn #doctorsofinstagram #obstetrics #gynecology #midwifery #testimonialsButton
-
We are BACK to normal business hours this week! 💙 Our team is working hard to reach out to all patients who were impacted by our closure last week. If your appointment was cancelled, you can expect to hear from one of our team members this week. Your patience with us is appreciated + and we can't wait to catch up with you soon! #women4womenhsv #women4womenobgyn #backinoffice #icestorm #weappreciateyouButton
-
T E M P S U R E E N V I Or as we like to refer to it, the IRON for your facial lines + wrinkles 🥵 TempSure Envi utilizes Radiofrequency energy to bypass HEAT through your skin's tissue in order to induce collagen + elastin production. TempSure reduces fine lines + wrinkles, immediately PLUMPS the skin, and leaves the skin with a natural GLOW. TempSure Envi is comfortable! Patient's indicate their treatments feel similar to a hot stone massage 💆 TempSure provides incredible results on its own, but we LOVE to add on Microneedling treatments to TempSure (and of course, skincare). To find out more information on TempSure, or curious about consulting with us, DM us and we'll send you available openings ❤ 💻 women4womenhsv.com 📱256-759-9269 #women4womenobgyn #womeninmedicine #medicalspa #tempsureenvi #cynosure #noninvasive #antiaging #tightskin #skincareguru #huntsvilleskin #huntsvilleal #ihearthsv #sohsv #ZOskinhealth #skinmedica #aesthetician #injector #botox #fillerButton
-
Uberlube is a luxurious silicone based lubricant with trace amounts of vitamin E ✨ Silky smooth + never sticky + no nasty, unnecessary ingredients. Uberlube does not harbor bacteria + yeast + or mold. 50 mls 👉🏼 $20 100 mls 👉🏼 $32 Shop using the link in our bio 🫶 #women4womenhsv #sohsv #uberlube #personallubricant #lube #obgyn #obstetrics #gynecology #health #doctorsofinstagramButton
-
January is Cervical Cancer Awareness Month 💙 Cervical cancer is a type of cancer that develops in a woman's cervix. It's important to get screenings annually to detect for cervical cancer. Have you scheduled your annual screenings for 2024? Early detection screenings can save your life. Don't wait! Schedule today! #women4womenobgyn #cervicalcancer #womeninmedicine #obgyn #gynecology #annualscreenings #earlydetection #ihearthsv #midwifery #wellness #aesthetics #obstetrics #gynecolgyButton
-
Due to hazardous road conditions, Women4Women OBGYN will be closed tomorrow, Thursday January 18th. If you have a scheduled appointment, someone will be in contact with you to reschedule your visit. We will continue to keep you informed on when we reopen. Stay safe + warm!Button
-
Due to hazardous road conditions, Women4Women OBGYN will be closed tomorrow, Wednesday January 17th. If you have an appointment, someone will be in touch to reschedule your visit. Stay safe and warm! 💙Button
-
We've been keeping a secret! Our Midwifery team grew! 👋 Meet our newest Certified Nurse Midwife, Lindsay Rossetti! Lindsay recently moved to Huntsville from Tupelo, Mississippi in order to fulfill her dream of engaging in the full time practice of midwifery with the midwives and physicians at Women4Women OBGYN. Lindsay is looking forward to using her knowledge and skills to bring dignity and worth to every individual she has the joy to encounter with Women4Women OBGYN! She is passionate about shared decision-making and evidence-based practice while supporting physiologic labor and birth, postpartum, and later stages of women’s healthcare. Lindsay and her husband, Benjamin, have three children, Isaac, Jude, and Evelyn, and two dogs, Captain and Rosie. They enjoy traveling, hiking, and beach trips with family. They look forward to exploring Huntsville and making it home! Join us in giving Lindsay a warm welcome to Huntsville! #women4womenobgyn #midwifery #midwives #certifiednursemidwife #obgyn #gynecology #obstetrics #holisticmedicine #rocketcity #iherthsv #HuntsvilleAL #sohsv #labor #birth #postpartum #takingcareofwomenButton
-
UPDATE: Our office will be CLOSED tomorrow due to inclement weather. If you have an appointment, someone will be in touch to reschedule. Stay safe + warm!Button
-
Today, we hope you take the time to reflect on the work that still needs to be done for racial equality + civil rights issues in our country. In honor of Martin Luther King Jr., our office is closed today 🖤 #women4womenobgyn #martinlutherkingjr #MLKday #racialequality #justice #huntsville #ihearthsv #sohsvButton
-
I L L U M I N A T I N G AOX + DAILY P O W E R DEFENSE Let’s talk about the crowd favorites today 👇🏻 As we age, our skin loses it’s ability to withstand environmental pollution and repair itself. Oxidative stress from external and internal factors can accumulate to cause low-grade inflammation that depletes skin’s health. Low-grade inflammation, or as we like to call it “inflammation aging, or inflammaging” leads to premature signs of aging. In the skin this can look like dehydration, fine lines, wrinkles, dullness, and uneven tone. Enter in a powerhouse solution, Illuminating AOX and Daily Power Defense RICH in antioxidants, specialized enzymes, plant stem cells + weightless barrier-builders, Illuminating AOX + Daily Power Defense work synnergetically to withstand the impact of environmental stressors. ILLUMINATING AOX — concentrated antioxidant serum that provides a luminous + soft finish to BLUR imperfections DAILY POWER DEFENSE — advanced serum that strengthens the skin’s protective barrier to restore the skin Ready to add these into your daily regimen? DM us for consultation availability! #Botox #Filler #ZOSkinHealth #ZOGlow #ZOskincare #skincareroutine #skincareguru #skinhealth #antioxidant #huntsvilleskin #huntsvilleal #rocketcity #nashvilleskin #fayetville #owenscrossroads #hazelgreen #madisonalButton
-
Tiny but mighty, the SkinPen Microneedling device treats mild to moderate fine lines, wrinkles, acne scars, texture irregularities, and discoloration. It is gentle, yet effective, with results being seen in as little as 5 days 🫶 All month, we're giving a travel size skincare kit including a cleanser, exfoliating polish, and complexion renewal pads to all facial aesthetic treatments! 📱256-759-9269 💻 women4womenhsv.com #women4womenhsv #sohsv #microneedling #skinpen #huntsvilleskin #nashvilleskin #fayetvilletn #botox #filler #zoskinhealth #skinmedica #medicalgradeskincare #skintreatmentsButton
-
DiamondGlow SERUMS + SKINCARE 🫶 One of the many benefits of the DiamondGlow facial is the ability to continue targeted treatments at HOME. There are FIVE serums on the DiamondGlow and a SkinMedica product to MATCH it! What's your favorite DiamondGlow Matchup?! #DiamondGlow #ElevateyourSkincare #Glow #skincareglow #glasskin #ihearthsv #hellohsv #allerganaesthetics #matchups #RevealYourRadiance #skinmedicaskincare #huntsvilleskinButton
-
What better way to kick off January than with a refined routine?! 😉 Every Facial Aesthetic treatment this month gets a free cleanser + polish + toner pads. Don’t miss out! #women4womenobgyn #medspa #healthcare #aesthetics #wellness #midwifery #zoskinheath #diamondglow #botox #fillerButton
-
Lumecca is a gentle, pigment-reducing intense pulse light treatment designed to target stubborn dark spots + vascular lesions + redness ⚡️ Clear skin is right around the corner with your F R E E consultation 🙌 #Women4WomenOBGYN #womeninmedicine #sohsv #hellohuntsville #ihearthsv #sohsv #huntsvilleskin #skincareguruButton
-
The time between Christmas and New Year’s can bring a lot of happiness, but sometimes the sick season can come along with it 😷 Today, we’re sharing our top 6 tips on practical things you can do to stay healthy during sick season ⬇️ * Eat nutrient dense meals ➡️ lots of vegetables, protein rich foods, and fiber will help to support your immune system * Increase your hydration ➡️ drinking more water helps to flush toxins out of your body. * WASH YOUR HANDS ➡️ Washing your hands will get rid of germs, protecting you and those around you! * Cover your cough ➡️ Prevent the spread of illness by covering your cough + sneezes. * Take a Vitamin C or Zinc Supplement ➡️ Vitamin C or Zinc can help boost immunity to potentially prevent sickness * Get adequate rest ➡️ Your body needs REST to stay healthy! Aim for 8 hours of sleep each night. 🤰🏼 For pregnant patients ➡️ did you know that we have a resource on our website for safe over-the-counter medications you can take during pregnancy to help with cold + flu symptoms? We have this linked in our bio for you! #womwn4womenobgyn #hellohuntsville #ihearthsv #sicknesstips #bewell #getwellsoon #coldandfluseason #wellness #huntsville #ihearthsvButton
-
Ready to join our club? 😉 You asked, and we delivered. The DiamondClub is officially L I V E 💎 The DiamondClub is an exclusive club for our most popular facial service: The DiamondGlow. Here's how it works 👇🏻 💎 A year of DiamondGlows (or 12 DiamondGlow Facials) for $1,440 ($120 per treatment vs. $200 retail). 💎 DiamondClub members receive 10% off Aesthetic Services and 15% off product for the entire 12 months 💎 25 spots so HURRY BOOKING + QUESTIONS 👇🏻 📱256-759-9269 💻 women4womenhsv.com #womeninmedicine #medspa #diamondglow #botox #medicalaesthetics #huntsville #ihearthsv #sohsv #hellohsvButton
-
Happy New Year from all of us at Women4Women OBGYN! May your 2024 be full of happiness, joy, and peace 💙 #women4womenobgyn #HappyNewYear #newyearsday #newyearnewgoals #huntsvilleal #ihearthsv #sohsv #hellohsv #rocketcityButton
-
And that’s a wrap on 2023! Whatever this year held for you, may the highs and lows fuel your path forward into an abundant 2024 💙 Cheers to an incredible year! We can’t wait to journey with all of you in the New Year! #newyear #newyearrecap #2024 #huntsville #newyearseve #ihearthsv #hellohuntsville #womwn4womenobgyn #midwifery #wellness #aestheticsButton
-
Repeat after me: skincare + in office treatments go hand in hand 🖐️ The DiamondGlow deeply cleans the skin through exfoliation and extraction of every single pore. By removing dead skin cells, we can revitalize dull, irritated skin to skin the GLOWS. The proof is in the pudding 🙃 Ready to see what comes out of your skin? 🫣 #womwn4womenobgyn #diamondglow #diamondglowfacial #satisfying #facial #skincare #hellohuntsville #ihearthsv #sohsvButton
-
From our Women4Women Family to you and yours, Merry Christmas 💚 #merrychristmas #Christmastime #happyholidays #Christmas #ihearthsv #sohsv #hellohsv #huntsvilleal #rocketcityButton
-
This year for Christmas, our Women4Women Family gathered goodies to donate to The Caring House ❤️ The Caring House is a non-profit organization located in Huntsville that serves children 3-18 who are mourning the death of a loved one. At any age, the loss of a loved one is detrimental. The Caring House recognizes that children can become “the forgotten mourners.” The Caring House is a safe haven for children to process their grief in order to heal in a healthy way. It is a quaint, cozy, and inviting space. The caring house provides physical and creative play, art expression, sharing and listening, expressing feelings, and learning about the grieving process. The Caring House is truly a unique and incredible space for children and young teens ❤️ Last night, we got to drop off our goodie basket full of arts and craft supplies gift cards, and even got to tour the Caring House! We feel so grateful to be able to serve the Caring House this year ❤️ #women4womenhsv #huntsvillehospitalfoundation #ihearthsv #sohsv #hellohsv #rocketcity #thecaringhouse #grief #children #counseling #nonprofit #christmasgivingButton
-
Sharing O U R results 💙 Our Aesthetics Manager, Krystina, and her results after O N E year on a custom ZO protocol. It didn't happen overnight for me, and it took a whole lot longer than 4-6 weeks to clear my skin. In fact, these pictures are taken almost one year apart. It's a dichotomy of painful and proud looking back at pictures of what my skin looked like. Acne lesions are equal parts physically painful (if you know, you know) and mentally exhausting. It's tough to look in the mirror and not recognize yourself. These before + afters go a lot deeper than what meets the eye. Yes, we can all see the reduction in lesions, inflammation, tighter skin, and a hydrated skin barrier but what doesn't meet the eye is the work done to control stressors in my life, diet changes, lifestyle changes, prioritizing sleep, water intake, less screen time, and I could go on and on and on. Treating acne, or any skin condition for that matter, is finding the balance of BOTH. If you feel like you're at a loss with your acne, or just the current state of your skin in general, believe me when I say I KNOW how you feel. If you need guidance with your skin, our Aesthetic's Team is here for you every step of the way. If you're ready to start your journey to clear skin, we'd love to chat with you. You can DM us "start" and I will be in touch with you on next steps 💙 #women4women #acne #ZOSkinHealth #HuntsvilleAL #fayetvilletn #nashvilleskin #skincare #treatingacne #madisonal #owenscrossroads #aesthetics #wellness #medspa #medicalaesthetics #cosmeticmedicine #beforeandafters #skinbeforeandafterButton
-
It's the last week to SHOP before the Holiday! 🎁 If you're in a need of a last minute gift or stocking stuffer, don't panic! We've curated a list of stocking stuffer ideas for the skincare guru in your life 🫶 All of these stocking stuffers + more are linked in our online store! DM us with any questions. Thursday is the LAST DAY for product pickup and shipment. This includes Gift Cards as well 💚 💻 women4womenhsv.com 📱256-759-9269 #women4womenhsv #womeninmedicine #holiday #merrychristmas #stockingstufferideas #giftguide #skincarelovers #skincaregifts #ihearthsv #happyholidays #hellohsv #sohsvButton
-
Merry + Bright at Women4Women! We love getting in the Holiday spirit, especially with our Christmas attire 🎄Button
-
Not just a reminder, but a fact! 💎 One of our favorite parts of the Holiday season is having the opportunity to get our sweet patients GLOWING for their holiday events. The DiamondGlow is a general dermabrasion device that simultaneously exfoliates, extracts, and infuses the skin with advanced SkinMedica serums. After treatment, your skin will literally GLOW from the inside out 🤩 Our Aesthetics schedule is currently full for the weeks leading up to Christmas + we are nearly full for New Years. To get on a waitlist or check availability for New Years, please call our office 👇 💻 women4womenhsv.com 📱256-759-9269 #DiamondGlow #RevealYourRadiance #DiamondTip #DiamondTechnology #ProInfusion #PoweredbySkinMedica #FacialTreatment #Glow #Facial #Glowyskin #skingoals #huntsvilleal #ihearthsv #hellohsv #sohsv #facialtreatment #huntsvilleskin #rocketcityButton
-
ZO SkinHealth G I F T SETS at Women4Women! Let’s face it (literally 😉) — the only kind of Chrsitmas we want is a blue Christmas from ZO + we have the PERFECT Holiday Gift Sets to make that happen! 💙 Our exclsuive gift sets are curated to make your holiday shopping easy (even if you’re treating yourself)! Each gift sets includes FULL SIZE ZO Product, is packaged in a gift box, and priced at a bundle deal. THE WINTER ESSENTIALS ($215) 👉🏼 Balancing cleansing emulsion, hydrating cream, and sheer fluid SPF. This gift set is ideal for those with dry, red, and sensitive skin. THE SIGNATURE GLOW (485) 👉🏼 If you’ve heard the buzz about ’The ZO Glow,’ and you’re ready to take the leap into ZO, then this gift set is for you 😉 This git set includes Illuminating AOX, Growth Factor Eye Serum, Wrinkle + Texture Repair, and Gel SPF RADIANCE RENEWERS ($230) 👉🏼 desiring radiant, glass skin? Enter in Radiance renewers gift set. This gift set includes complexion renewal pads, exfoliation accelerator, and retinol skin brightener. To purchase 👇🏻 👩💻 link in our bio to our online store. We ship! 📞 256-759-9269 to place an order #women4womenobgyn #womeninmedicine #medspa #ZOskinhealth #ZOglow #ZOskincare #ZO #Zoproducts #christmasgifts #skincaregifts #holidaygiftguide #tistheseason #happyholidays #huntsvilleal #huntsvilleskin #ihearthsv #sohsvButton
-
Hurry! 15% OFF now means 15% OFF your future Aesthetic service + procedure + product. Gift cards are 15% off through the end of the month. Don't miss out! Link in our bio to purchase or call our office 🎁 #women4womenobgyn #womeninmedicine #giftcards #giftcardsale #sohsv #hellohsv #skincare #aesthetics #wellness #giftguide #botoxfiller #botoxtreatment #FillerMagic #zoskincare #SkinMedicaSkinCare #morpheus8face #IPL #diamondglowfacial #laserhairremovalButton
-
Ringing in the Holiday Season with the most incredible team ever! 🎄 We love any opportunity to get out of our scrubs, get dressed up, and have an evening out with the people that make our practice so special ❤️ From all of us at Women4Women, Happy Holidays! #team #merrychristmas #women4womenobyn #womeninmedicine #holidays #huntsville #ihearthsvButton
-
Our Holiday Promotions are LIVE! ✨🎁 💚 15% OFF Gift Cards ALL month long! Minimum value of $100 required. Gift cards can be redeemed on any Aesthetic service + procedure. ❤️ Gift with Purchase 👉 Spend $250 on ZO SkinHealth and receive a travel size Daily Power Defense + 3 Skin Brightening Sheet Masks. Spend $350 on SkinMedica and receive a FREE SkinMedica Mini Method ($125 value). To purchase 👇 💻 visit our online store! Link is in our bio. 📱 Call our office! 256-759-9269 #women4womenobgyn #womeninmedicine #sohsv #holidaypromotions #ZOglow #skinhealth #skinmedica #huntsville #hellohsv #nashville #nashvilleskin #skincarelover #december #christmaslistButton
-
You know the phrase “you can’t have one without the other?” That same analogy can be used for seeing results in Aesthetics. - You can’t sustain long term results of in office treatments without medical grade skincare. - You can’t enhance the results of Botox and Filler without medical grade skincare. - Medical grade skincare can only get your results so far without the addition of injectables and in office treatments. This precious patient of ours has been seeing our Aesthetics team for a little over 2 years. She started seeing us for her Botox treatments a few years before her 30th birthday for preventative aging. It took a lot of trust building to get her to committ to protocol of skincare and in-office treatments, but boy is she SO happy she did. LOOK at how HEALTHY her skin looks! AND she’s older in the picture on the right. If you needed proof that you can gracefully age, this is it. We adore this patient so much and we’re SO happy for her! We're so grateful that she has put her trust in us + can't wait to see how her skin continues to transform over the next 2 years 💙 #women4womenobgyn #womeninmedicine #medspa #ZOSkinHealth #botox #filler #injectables #skincareroutine #resultsdriven #skincareglow #ZOglow #huntsvilleal #huntsvilleskin #diamondglow #microneedling #skinpen #ihearthsv #sohsv #nashvilleskin #fayetvilletn #madisonal #athensalButton
-
Today we're chatting the Holy Grail retinol for aging + acne 👇 Meet Wrinkle + Texture Repair 💙 Wrinkle + Texture repair is a .5% retinol that targets deep lines, improves the texture of the skin and is ideal for patients who have rough, "leathery" skin that is sun damaged and also great for patients with acne. Wrinkle + Texture repair is formulated with exclusive ZO delivery systems that enhance collagen + elastin production, helps prevent future signs of aging, and protect against cellular damage. Wrinkle + Texture helps support the structural strain between the dermis + epidermis which results in LESS sagging of the skin, and promotes nutrient flow which promotes a natural skin renewal cycle. Ready to chat about incorporating a retinol into your routine? DM us to get started! 💻 women4womenhsv.com 📱255-759-9269 #womeninmedicine #ZOskinhealth #retinol #skinhealth #zoglow #skincare #skincareroutine #preventativeagin #aginggracefully #sohsv #hellohuntsville #huntsvilleskin #skincareishealthcareButton
-
Repping Women4Women at the Action Summit 2023/COP 28 in Dubai! #cop28dubai #climatechangeButton
-
Winter essentials we are loving ✨ These cold, winter temperatures are often really tough on our skin! If you’re having a difficult time with dry, flaking, “snake” skin, then it may be time to consider adding some of these items into your regimen ⬇️ HYDRATING CLEANSER — soothes + calms sensitive skin. COMPLEXION RENEWAL PADS — exfoliates pore clogging dead skin cells in order to replenish hydration. HYDRATING CREAM — encourages cell proliferation and skin renewal to enhance natural healing, reduce itching, and minimize inflammation + redness. GEL SPF — plant stem cell complexes deliver environmental protection + smooth the skin. Complexion smoothing finish, hydrating, and blends with all skin tones. Ready to start your journey to clear, healthy skin? Call or DM us for a consult appointment 💙 #skincare #womwn4womenobgyn #dryskinremedies #womeninmedicine #zoskinhealth #huntsville #fayetvilletn #nashvilleskin #madisonalButton
-
BLACK FRIDAY IS HERE! ✨ NOW through Monday, enjoy 20% OFF all online orders! Shop through the link in our bio and use code "BLACKFRIDAY" to redeem your discount! 🎁 GIFT WITH PURCHASE 👇 Orders of $350+ on SkinMedica will receive a SkinMedica Mini Method ($125 value) and orders of $350+ on ZO SkinHealth will receive a travel size Daily Power Defense. Now is a great time to stock up or get Christmas presents! #women4womenobgyn #womeninmedicine #blackfriday #blackfridaysale #medspa #zoskinhealth #allerganaesthetics #skinmedicaskincare #sales #deals #huntsville #smallbusiness #rocketcity #huntsvillealButton
-
From our Women4Women Family to you and yours, Happy Thanksgiving! We hope your day is spent with all those that you love 🤍 #women4womenobgyn #women4women #thanksgiving #happythanksgiving #holiday #sohsv #hellohsv #sohsv #ihearthsv #rocketcityButton
-
Happy Thanksgiving Week! We will have condensed hours this week 👇 ⏰ We are closing at 12:00 p.m. TOMORROW, Wednesday November 22nd and will be CLOSED for Thanksgiving. We hope you all have a wonderful Thanksgiving Holiday! #women4womenobgyn #womeninmedicine #holidayhours #happythanksgiving #ihearthsv #sohsv #rocketcityButton
-
A few snapshots from our staff at our Holiday Open House 🎉 We had a record breaking event this year and a simple “thank you” does not even begin to cover how grateful we are to each of you and your support of our Aesthetic’s Practice! If you needed time to think about a product or service, or forgot to call, our promotions are extended until 5:00pm ☺️ And a huge shoutout to Lindsay with @sweetrootsphotography for photographing our events so well. Without her, we wouldn’t have pictures (these events get hectic) 🤪 Cheers to a wonderful kick start to the Holiday season + we are so eager for next year! #women4womenobgyn #womeninmedicine #holidayopenhouse #aesthetics #wellness #medspa #huntsvilleal #ihearthsv #sohsvButton
-
✨ IT'S OPEN HOUSE DAY!! ✨ Our promotions are LIVE and we are SO excited to see all of you TONIGHT! Can't make it?! You can call to place an order 😃 OPEN HOUSE DETAILS 👇 📅 TONIGHT, Thursday November 16th ⏰ 6:00 p.m. - 8:00 p.m. 📍320 Pelham Avenue Suite 301 See ya'll soon! #women4womenobgyn #openhouseday #holidayevent #partyseason #womeninmedicine #medspa #aesthetics #botox #filler #skinpen #microneedling #IPL #morpheus8 #laserhairremoval #chemicalpeels #ZOSkincareButton
-
💥 ONE more sleep until our Holiday Open House! 💥 We can't believe our Holiday Open House is upon us and we are SO excited to kick off the Holiday season with each of you! Today, we are excited to launch our GIVEAWAYS of over $5,000 worth of services and products. Scroll through to see our raffles! Which one are you most excited for? To enter into each raffle, all you have to do is come to our event tomorrow! The details 👇 📅 Thursday, November 16th ⏰ 6:00 p.m. - 8:00 p.m. 📍320 Pelham Avenue Suite 301 RSVP'ing is not required, but we would love to know if you are coming! Link to RSVP is in our stories + bio ✨ #women4womenhsv #womeninmedicine #openhouse #botox #filler #microneedling #IPL #giveaway #HuntsvilleAL #ihearthsv #sohsv #northalabama #skincarelover #skincareguru #partytime #aesthetics #wellnes #medspaButton
-
🤩 T W O DAYS AWAY FROM OUR HOLIDAY OPEN HOUSE! 🤩 We are SO excited that we are officially 2 DAYS AWAY from our Open House! Today, we're chatting about the #1 non-invasive fat reduction system, CoolSculpting ❄️ Do you have stubborn pockets of fat that aren't going away with diet and exercise? Do you feel like you've done everything you can to get rid of the stubborn fat but nothing you try is working? Well, you're in good hands! CoolSculpting could be the the coolest solution for you 😎 CoolSculpting is FDA-cleared, safe and effective for the non-invasive removal of subcutaneous fat cells through controlled cooling. For the average patient, it takes 2 treatments + 4-6 weeks for optimal results (and there is no downtime) 🫶 Scroll through these before + after's of our own patients to see the results for yourself! OPEN HOUSE PROMOTION 👇 Cycles 1-4 👉 $500 ($1,000 savings) Cycles 5+ 👉 $400 ($1,750+ savings) OPEN HOUSE DETAILS 👇 📅 Thursday, November 16 ⏰ 6:00 - 8:00 p.m. 📍320 Pelham Avenue Suite 301 We can't wait to see ya'll this THIS THURSDAY! #coolsculpting #coolresults #allerganaesthetics #fatreduction #huntsvilleal #ihearthsv #sohsv #allerganaesthetics #botox #filler #savingsButton
-
We can't let this week pass us by without wishing a very Happy Nurse Practitioner's Week to our very own, Elizabeth Irby! Elizabeth wears many hats around our office: she is a caring provider and tender-hearted leader in her role as Nurse Manager. She tackles all her responsibilities exceedingly well, while doing so with such integrity. Elizabeth, THANK YOU would never be enough for ALL that you do for our office. Thank you for taking such great care of your patients + our staff! We love you! 🫶 #women4womenobgyn #nursepractioner #womenshealth #obgyn #obstetrics #gynecology #womeninmedicine #Huntsville #ihearthsv #rocketcity #hellohsv #nursesButton
-
IT'S OPEN HOUSE WEEK! 🙌 We can hardly believe that we are just 3 sleeps away from our Holiday Open House! Today, we're chatting all things SKIN TIGHTENING 🥳 As we age, we begin to notice the effects of collagen and elastin breakdown (aka gravity de-friends us 🥴). Your collagen + elastin fibers help maintain the overall structure of the skin (think: collagen + elastin are the backbone of my skin). Around the age of 30, our bodies begin to degenerate collagen and elastin production. When we experience this breakdown, we start to notice some signs of aging. This could look like 👇 Fine lines, wrinkles, skin laxity, or tone + texture irregularities. To COMBAT the effects of collagen + elastin breakdown, we welcome Morpheus8, TempSure Envi, and SkinPen Microneedling to that chat 👊 Morpheus8 👉 RadioFrequency Microneedling that works DEEP into your tissue. Morphues8 is able to penetrate your skin's tissue up to 4mm and the RF signal is sent 1mm deeper than the depth of the needles. While Microneedling builds collagen, RadioFrequency energy TIGHTENS the skin. The results of Morpheus speak for themselves! TempSure Envi 👉 topical RadioFrequency to tighten the skin without downtime and totally non-invasive! TempSure Enviros heats the surface of the skin to stimulate collagen to breakdown and reproduce. TempSure Enviros feels like a hot stone massage + results can be noticed immediately after 🥳 SkinPen 👉 standard Microneedling without energy (and a fan favorite)! This is our go-to for our Pregnant + Postpartum mama's or those without significant laxity. SkinPen microneedling penetrates the skin up to 2.5mm and changes the game for tone + texture of the skin, acne scarring, and clarity of the skin. Scroll through to see the results for yourself 🥰 OPEN HOSUE DEALS 👉 20% OFF PACKAGES OF 3+! Come hang out with us 👇 📅 Thursday, November 16th 📍 320 Pelham Avenue Suite 301 ⏰ 6:00 - 8:00 p.m. #women4womenhsv #womeninmedicine #skintightening #results #morpheus8 #tempsurenevi #sohsv #huntsville #ihearthsv #hellohsv #aesthetics #wellness #skinpen #microneedling #skincare #skinlover #medspa #madisonal #huntsvilleskin #nashvilleskin #fayetvilletnButton
-
🥳 We are just FOUR days away from our Holiday Open House 🥳 Today we're talking all things DIAMONDGLOW 💎 The DiamondGlow is hands down the most loved service among our patients + staff. Using a 3x1 technique, the DiamondGlow simultaneously exfoliates the skin, extracts EVERY SINGLE PORE and then infuses the skin with SkinMedica's Advanced Pro-Infusion Serums. The DiamondGlow is luxurious, comfortable, and effective. The DiamondGlow is 100% customizable (which is our favorite part about it)! Wherever your skin is, we have a serum to meet it where it's at 👏🏻 At our Holiday Open House THIS THURSDAY, we're taking $50 OFF 1 treatment OR 20% off packages of 3 or 5 👇 1 treatment 👉 $150 (retail $200) 3 treatments 👉 $432($144 per treatment). Retail value of $540. 5 treatments 👉 $600 ($120 per treatment). Retail value of $750. Who's ready to GLOW like a DIAMOND?! Open House Details 👇🏻 📅 Thursday, November 16th ⏰ 6:00 - 8:00 p.m. 📍 320 Pelham Avenue Suite 301 #women4womenobgyn #womeninmedicine #huntsvilleal #ihearthsv #sohsv #hellohsv #huntsvillevent #diamondglow #allerganaesthetics #madisonal #huntsvilleskin #skincarelover #botox #filler #nashvilleskin #rocketcity #aesthetics #medspaeventButton
-
🥳 F I V E DAYS AWAY FROM OUR HOLIDAY OPEN HOUSE 🥳 Today, we're talking about the fan favorite, SKINCARE PRODUCTS! Our Aesthetics Team is committed to providing products that drive and enhance results + support in office treatments. We know that good, quality, medical grade skincare products assist in achieving results, whether you make the choice to do in office treatments or not. In fact, our very step before we pursue an in office treatment protocol is curating a custom skincare protocol for you. Achieving good skin health is WORK + it takes TIME. We are here for you every step of the way until your skin is CLEAR 💙 Scroll through a few of these before + after's of some of our skincare patients -- we are so proud of each of them and their commitment they've made to themselves! OPEN HOUSE PRODUCT PROMOTION 👉 30% OFF! When you spend $400+, you'll qualify for a FREE chemical peel 🫶 HOLIDAY OPEN HOUSE 📍320 Pelham Avenue Suite 301 📅 Thursday, November 16th ⏰ 6:00 pm - 8:00 p.m. #Women4womenobgyn #womeninmedicine #takingcareofwomen #skincare #ZOSkinhealth #SkinMedica #skincarelovers #sohsv #hellohsv #ihearthsv #huntsville #huntsvillevent #madisonal #fayetvilletn #nashvilleskin #skinhealthButton
-
👏 6 DAYS UNTIL OUR HOLIDAY OPEN HOUSE 👏 Today, let's chat about IPL! IPL, otherwise known as Intense Pulsed Light, targets unwanted pigment in your skin, redness, and vascular lesions. Our IPL device, the Lumecca, is the most powerful IPL device available (maximum results? Yes please)! After just ONE treatment, our patients indicate significant improvements in the overall clarity + complexion of their skin. Scroll through some of our incredible before + after's to see for yourself! HOLIDAY OPEN HOUSE PROMOTION 👉 20% OFF PACKAGES OF 3+ 🤩 Full Face package of 3 👉 $960 at our Open House (over $500 savings) 🤩 OPEN HOUSE DETAILS 👇 📍320 Pelham Avenue Suite 301 ⏰ 6:00 - 8:00 p.m. 📅 Thursday, November 16 #women4womenhsv #women4womenobgyn #openhouse #aesthetics #medicalaesthetics #medspa #madisonal #huntsvilleal #ihearhsv #huntsvilleevent #IPL #savings #skincare #skincarelover #huntsvilleskin #sohsvButton
-
"IT'S THE FINAL COUNTDOWN!" 🎶 We can't believe we are just SEVEN days away from our Holiday Open House! We are excited to launch our promotions TOMORROW! What are package or product are you most looking forward to? We can't wait to kickstart the Holiday season with all of YOU! Grab your friends + come enjoy a night out with us. 📅 Thursday, November 16th ⌚️ 6:00 p.m. - 8:00 p.m. 📍 320 Pelham Avenue Suite 301 📱256-759-9269 #women4womenobgyn #openhouse #medicalaesthetics #botox #filler #skintightening #microneedling #medicalgradeskincare #zoskinhealth #skinmedica #skincarelover #deals #promotions #ihearthsv #hellohsv #huntsvilleeventButton
-
MEDICAL GRADE SKINCARE 💥 You've probably heard about it, but what is ALL the hype about it? All you need to know 👇 Medical grade skincare is superior to over-the-counter or professional skincare because product efficacy is much higher. So, what is high product efficacy? Medical grade products have higher standards of manufacturing, testing, regulations, and have a the highest potency of ingredients used. Medical grade skincare is backed by scientific AND clinical research. With higher potency of ingredients, products have increased absorption and decreased sensitivities (excluding some products like retinol), and BETTER more LASTING results 💥 AT our Holiday Open House NEXT THURSDAY you will have the opportunity to learn more about medical grade skincare + get all your questions answered (and we have a realllllly good deal lined up for you 💥) 📅 Thursday, November 16 from 6:00 - 8:00 📍320 Pelham Avenue Suite 301 📱256-759-9269 💻 women4womenhsv.com #women4womenobgyn #womeninmedicine #takingcareofwomen #medicalaesthetics #medspa #ZOskinhealth #skinmedical #skincarelovers #ihearthsv #sohsv #huntsvilleal #openhouse #allerganaesthetics #madisonALButton
-
🥳 HAPPY NOVEMBER! It's PARTY MONTH for us here at Women4Women OBGYN! Our Holiday Open House is nearly 2 WEEKS AWAY and we can hardly wait! JOIN US November 16th from 6:00 p.m. - 8:00 p.m. for champagne, charcuterie, and FUN! We'll even have a SELFIE BOOTH 🤩 📍320 Pelham Avenue Suite 301 Huntsville, AL 35801 NEXT WEEK we are launching our promotions + our giveaways! Who's ready to party?! Let us know you're coming by visiting the link in our bio! #women4womenhsv #womeninmedicine #ihearthsv #huntsvilleevents #rocketcity #aesthetics #medspa #medicalaesthetics #openhouse #selfies #hellohsv #HuntsvilleALButton
-
Happy Halloween from all the "ghouls" at Wmen4Women OBGYN! We hope you have a SPOOKTACULAR day 👻 #women4womenobgyn #happyhalloween #spookyseason #spooky #huntsvilleal #ihearthsv #hellohsvButton
-
Results that are SPOOKTACULAR 👻🫶 We are over the moon excited for this sweet patient of ours and the results she has achieved with the CoolSculpting fat reduction system. Looking through her before and after gallery, she exclaimed, "WOW! I knew I was experiencing results, but seeing them side by side is surreal." We couldn't be more thrilled for her! Are you ready to tackle your body contouring goals? You're just ONE free consult away 🖤 Scheduling 👇 📱256-759-9269 💻 women4womenhsv.com #women4womenhsv #women4womenobgyn #womeninmedicine #bodycontouring #coolsculpting #coolresults #allerganaesthetics #hellohsv #ihearthsv #sohsv #huntsvilleskinButton
-
A key term in our DiamondGlow dictionary 💎 The Diamond tips allow us to customize your treatment for your specific skin concerns + skin's sensitivity. There is truly no glow like the DiamondGLOW 💎 Using a 3 in 1 technique, the DiamondGlow simultaneously exfoliates, extracts, and infuses the skin with advanced SkinMedica serums. Patient's can achieve correction of fine lines + wrinkles, discoloration, acne, and much more. There's a reason DiamondGlow is a favorite among our patients! Let's get glowing 👇 📱256-759-9269 💻 women4womenhsv.com #Women4WomenOBGYN #DiamondGlow #RevealYourRadiance #DiamondTip #DiamondTechnology #ProInfusion #PoweredbySkinMedica #FacialTreatment #Glow #Facial #GlowySkin #SkinGoals #Skincare #WomeninMedicine #Huntsvilleskin #ihearthsv #sohsvButton
-
MARK YOUR CALENDARS! 🥳📅 Our open house date is set + we are ready for YOU to get the BEST deals of the season on your favorite Aesthetic products + procedures. THE DETAILS: 📅 Thursday, November 16th from 6:00 p.m. - 8:00 p.m. 📍Women4Women OBGYN -- 320 Pelham Avenue Suite 301 Huntsville, AL 35801 (3rd floor of Bryant Bank) 👋 Let us know you're coming by registering through the link in our bio! https://www.eventbrite.ca/e/aesthetics-holiday-open-house-tickets-744467551487?aff=oddtdtcreator 🤩 STAY TUNED for more details on promotions, raffles, prizes, AND MORE FUN that we have in store! We cannot wait to welcome you into our clinic + kick off the Holiday season with all of YOU! Questions?! Message us! 💻 women4womenhsv.com 📱256-759-9269 #openhouse #aestheticsopenhouse #huntsvillevents #event #freehuntsvilleevent #ihearthsv #rocketcity #northalabama #skincare #ZOSkinHealth #SkinMedica #allerganaesthetics #botox #filler #huntsvillemedspa #holidaypartyButton
-
👀 SPOOK your DROOP with UpNeeq! UpNeeq is an FDA approved prescription eye drop that treats acquired ptosis, or "droopy eyelids." Taking effect in 15 minutes, UpNeeq lasts 8-10 hours and peaks at 2 hours. UpNeeq is 20% off this month! Don't let your droopy eyelids spook ya, get UpNeeq'd! #UpNeeq #eyelids #droopyeyelids #sohsv #aesthetics #wellness #medicalaesthetics #huntsvilleal #sohsv #eyecare #ihearthsv #womeninmedicine #northalabama #skincare #takingcareofwomenButton
-
Skincare advice that is SCARY 😫 BOOtiful skin + spooky good skincare advice is ONE free consultation appointment away! Call us to book! 📱256-759-9269 💻 women4womenhsv.com #women4womenhsv #hellohsv #skincareadvice #skincarelover #skincare #womeninmedicine #medspa #medicalaesthetics #ZOskinhealth #SkinMedica #medicalgradeskincare #HuntsvilleALButton
-
You've probably heard a lot of buzz about Vitamin C serum, but why is Vitamin C so important? We're glad you asked 😁 Vitamin C is an antioxidant serum. Antioxidants fight against free radicals found in our environment 👉 Free radicals = pollution (like sun exposure ☀️) = oxidative stress on the skin = inflammation and damaging the skin (think: aging and hyperpigmentation). Vitamin C acts as a shield against free radical damage! It protects the skin from oxidative stress that is caused by free radicals. Vitamin C prevents future pigment, corrects existing pigment, and can visibly brighten the skin ☺️ THIS MONTH you can get a travel size 10% Self Activating Vitamin C FREE with our "Ghost your Pigment" special 👉 3 full face IPL treatments for $1,000 ($500 savings) 🫶 Booking and questions 👇 📱256-759-9269 💻 women4womenhsv.com #women4womenobgyn #womeninmedicine #healthcare #skincare #medicalaesthetics #medspa #vitaminC #ZOSkinHealth #glowingskin #glow #fallskincare #ipl #huntsvilleal #ihearthsv #sohsv #hellohsvButton
-
Achieve FREAKY long lashes with Latisse! Latisse is FDA approved to treat inadequate eyelash growth. Latisse prolongs the active growth phase and is clinically proven to deliver lashes that are longer, fuller, and darker 🖤 You can get your hands on Latisse this month for 20% off! Call our office to purchase! 💻 women4womenhsv.com 📱 256-759-9269 #women4womenobgyn #womeninmedicine #latisse #freakylonglashes #eye #eyecare #skincare #eyelashes #eyelashserum #huntsvilleal #ihearthsv #sohsv #hellohsv #takecareofyou #EmpowermentButton
-
🎉 We have a BIG celebration today! We are wishing the happiest birthday to our wonderful Owner + Founder, Dr. Anne Marie Reidy! 🥳 We say it all the time, but Dr. Reidy truly excels in all that she does. She is caring, compassionate, generous, and thoughtful. She is an exceptional leader who is decisive, reliable, and accountable. She serves + advocates for her patients and staff exceedingly well while being a friend to all. Dr. Reidy, we are hopeful this next year is one for the books! Thank you for inspiring ALL those around you and for ALL that you do for your community. Join us in wishing Dr. Reidy a happy, happy, Birthday! #women4womenobgyn #womeninmedicine #birthday #happybirthday #itsyourbirthday #ihearthsv #hellohsvButton
-
Several staff members from our Women4Women family had the opportunity to attend the Tie the Ribbons luncheon benefitting Hudson Alpha’s Breast and Ovarian Cancer Program. This organization and the work they are doing is near and dear to our hearts 💙 Our very own Owner and Founder, Dr. Anne Marie Reidy, was chosen to speak on the importance of Information is Power for patients in our practice + community. Dr. Reidy was the only doctor from North Alabama selected to represent our amazing community. You can watch her interview through the link in our bio 💙 #women4womenobgyn #informationispower #genetictesting #womeninmedicine #takingcareofwomen #knowledgeispower #breastcancerawareness #ovariancancerButton
-
BEWARE of these 7 skincare sins 👻 Ready to achieve spooky good skin? Book a FREE consultation with our spooktacular Medical Aesthetician, Alex 👇 💻 women4womenhsv.com 📱256-759-9269 #women4womenobgyn #skincaresins #skincaretips #ZOSkinHealth #SkinMedica #glowingskin #skincaremistakes #huntsvilleal #ihearthsv #hellohsv #sohsv #huntsvilleskin #northalabamaskin #madisonskin #madisonalButton
-
We are obsessed with these results 🫶 We are blown away over these results after 3 treatments of SkinPen Microneedling. The overall clarity in the skin, reduction in pigment, and improvement in texture is incredible. Microneedling is literal magic ✨ Ready to achieve results for your skin that are magical? 👇 💻 women4womenhsv.com 📱256-859-9269 #women4womenhsv #womeninmedicine #takingcareofwomen #skinpen #microneedling #crownaesthetics #results #beforeandafter #huntsvilleal #huntsvilleskin #skincareloversButton
-
The only thing we're ghosting this season is our pigment 👻 IPL (Intense Pulsed Light) targets unwanted pigment in the skin. Vascular lesions, any redness in the skin, sun spots, and age spots can be treated with IPL. IPL season is in full swing + we don't want you to miss out! We brought back our most popular IPL promotion this month 👉 GHOST YOUR PIGMENT by receiving 3 full face IPL treatments AND a FREE Vitamin C Serum for $1,000 (orig. $1,500). Call us to book! 📱256-759-9269 💻 women4womenhsv.com #women4womenobgyn #womeninmedicine #IPL #intensepulsedlight #discoloration #hyperpigmentation #vitaminC #ZOskinhealth #skincareishealth #skincare #hellohsv #huntsvilleskin #ihearthsv #huntsvilleal #rocketcityButton
-
October is Breast Cancer Awareness Month! Today, let's chat about the 4 things you can do to screen for breast cancer 👇 1. Perform self breast exams 👉 the majority of breast cancers diagnosed are found by the patient. It's imperative to regularly check your breasts, or as we like to say "get to know them!" If you feel something, say something. 2. Schedule your annual exams 👉 staying on track with your annual screening appointments can be very beneficial for early detection. Detecting breast cancer early can positively impact treatment outcomes. 3. Get regular mammograms 👉 mammograms take an x-ray image of the breast. Mammograms are used for screening and diagnostic purposes. Make sure you have your yearly mammogram scheduled! 4. Know your family history 👉 knowledge is POWER! If you know you have a family history of breast cancer then you can make a plan for surveillance including baseline mammograms and genetic testing. 💻 women4womenhsv.com 📱256-759-9269 #women4womenhsv #womenshealth #breastcancer #BreastCancerAwarenessMonth #BreastCancerSurvivor #EarlyDetectionMatters #HuntsvilleAL #sohsv #hellohsv #rocketycity #womenshealthcare #doctorsofinstagramButton
-
We are HIRING! We are looking for a highly motivated, dependable, and compassionate Medical Assistant (MA) to join our clinical staff at Women4Women OBGYN. If you are passionate about building relationships, are a self starter, and love a fast paced environment this position is for you. - 1+ years of MA experience required. - OB/GYN experience preferred. - Position is PART TIME, Wednesday and Thursday with the potential for full time in the future. Our Women4Women Family is dynamic and team oriented. If you think you would be a good fit, please email your resume to Elizabeth at eirby@women4womenhsv.com #hiring #women4womenobgyn #joinourteam #huntsvillejobs #MedicalAssistantJobs #ihearthsv #huntsvilleal #rocketcity #healthcarejobs #wearehiringButton
-
Step inside a Chemical Peel treatment with our Medical Aesthetician, Alex! Chemicl Peels are used for the treatment of fine lines and wrinkles, discoloration in the skin, acne, scarring, and tone + texture irregularities. Chemical peels sound intimidating and scary, but we promise they aren’t! Chemical peels are offered at different depths; light, medium, and deep. If you’re new to chemical peels, we start with our light “no peel, peel” and move up from there. There’s nothing like that post chemical peel glow 👏🏻 🎃 SHED the DEAD this month 👉🏼 20% OFF packages of 3+ Chemical Peels (you can mix + match between peels)! Questions?! DM Us! Scheduling 👇🏻 👩💻 women4womenhsv.com 📞 256-759-9269 #women4womenobgyn #medspa #chemicalpeels #takingcareofwomen #medicalaesthetician #aesthetics #wellness #peelseason #glowingskin #skincarelovers #skinmedica #ZoSkinHealth #huntsvilleskin #ihearthsv #sohsv #hellohsv #huntsvilleskincareButton
-
Cool, cool, cool results 😎 Before + 6 weeks after 2 treatments to this patient's upper and lower abdomen with CoolSculpting. She's been working hard on her diet and exercise, but still had stubborn fat that would not budge. CoolSculpting to the rescue! CoolSculpting is designed to treat stubborn fat pockets that do not go away with diet and exercise. We are thrilled for this patient and so happy she entrusted us to help her meet her end goal! Ready to talk about your goals? Let's chat (it's free)👇 📱256-759-9269 💻 women4womenhsv.com #coolsculpting #fatremoval #nosurgery #nodowntime #huntsvilleal #ihearthsv #hellohsv #sohsv #women4womenobgn #womeninmedicine #aestheticmedicine #takingcareofwomen #womenshealth #madisonalButton
-
We are celebrating National Midwifery week this week! We feel so grateful to have FOUR wonderful Certified Nurse Midwives on our Provider Team. Tiffany, Sam, Diana, and Megan excel in providing the highest level of care for their patients; whether it be during their annual visits, throughout their pregnancy, and during the postpartum period. Our Certified Nurse Midwives only deliver in a hospital setting (Huntsville Hospital Women & Children) 🫶 Thank you, to our Midwifery Team, for ALL that you do. You each are an integral part of Women4Women and our practice vision would not be complete without you. Join us in celebrating our Midwives this week! #nationalmidwiferyweek #callthemidwife #midwifery #obstetrics #gynecology #women4womenobgyn #womeninmedicine #takingcareofwomem #holistichealth #huntsvilleal #rocketcity #babies #pregnancy #givingbirth #birthstory #birthplanButton
Contact
Phone: (256) 759-9269
320 Pelham Avenue SW, Suite 301
Huntsville, AL 35801
(On 3rd Floor, Enter on North side of Bryant Bank Building)
All Rights Reserved | Women4Women OBGYN
Privacy Policy | Site Design by Infinity Medical Marketing